Sensible (specific) heat and heat exchange without change of state. – EN
=
Sensible (specific) heat and heat exchange without change of state.
Calorimetry
Calorimetry refers to the determination of the amounts of heat absorbed or released in various thermal phenomena.
Thermal energy
Consider two bodies A and B made of the same material , but with different masses (m A > m B ), and at the same temperature.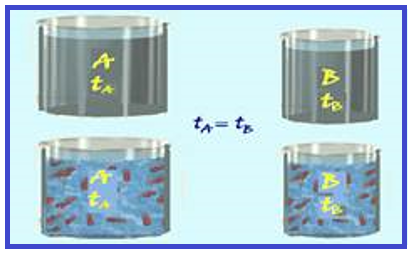
Since t A = t B, the vibratory movement (kinetic energy) of the molecules of A and B are equal , but as m A > m B , body A has a greater quantity of molecules than B.
Thus, when you add up the kinetic energy of all the molecules of A, you will get a value greater than the sum of the energy of all the molecules of B, making the thermal energy of A greater than the thermal energy of B , even though they are at the same temperature.
Heat
When two bodies A and B at different temperatures and thermally isolated are placed in contact , it is seen that the colder one heats up and the hotter one cools down , until they have the same temperature (thermal equilibrium temperature).
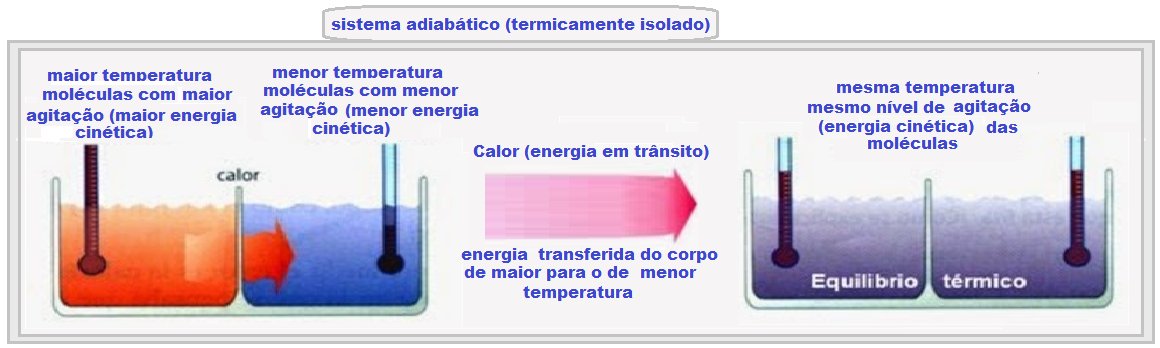
In order to establish thermal equilibrium between the two bodies, a certain amount of energy must be transferred from the body with the higher temperature to the body with the lower temperature.
This energy in transit is called heat.
What you should know, information and tips
![]()
Heat is thermal energy in transit.
![]()
It cannot be said that a body has heat , but that it has thermal energy.
Heat units
Since heat is thermal energy in transit, in the International System of Units (SI) , heat and energy have the same unit, the joule (J).
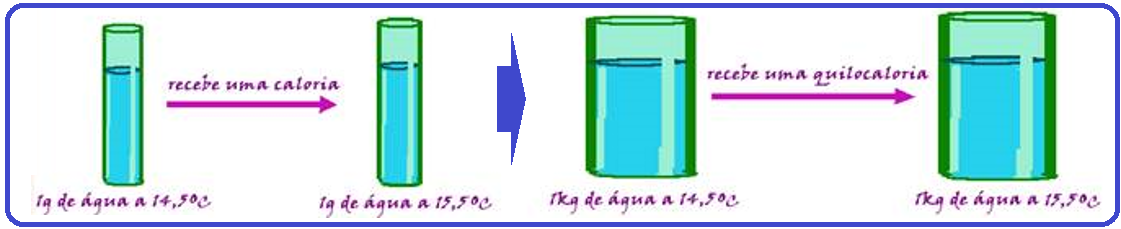
However, you can also use calorie (cal) or kilocalorie (kcal), such that 1 kcal = 1,000cal.
Definition of Calorie or cal: amount of heat required to raise the temperature of 1g of water from 14.5 o C to 15.5 o C, that is, 1 o C.
![]()
1 kcal = 1,000 calories.
![]()
Heat propagation processes
Heat propagates whenever there is a difference in temperature between two or more bodies .
This propagation always occurs from the body with the highest temperature to the body with the lowest temperature.
This transfer occurs through three processes
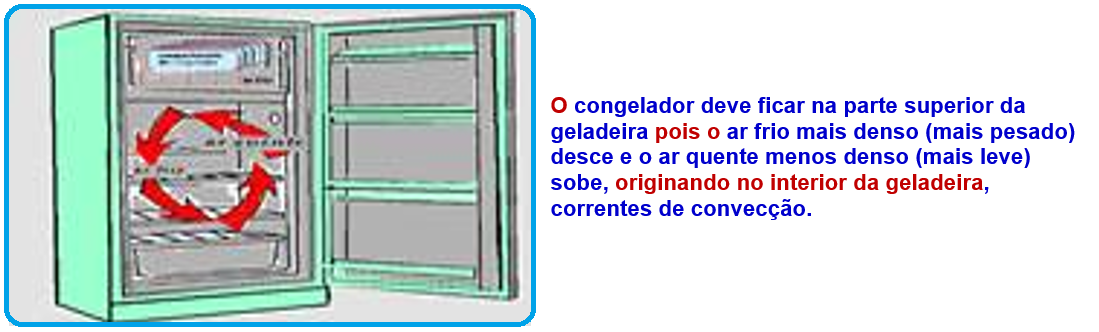
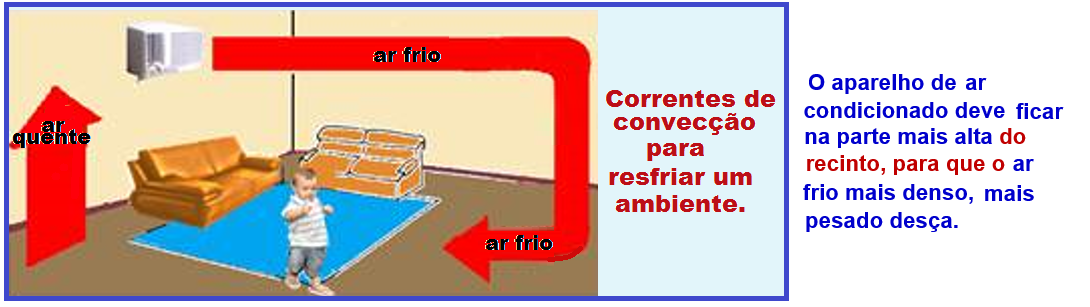
Thermal convection
This is the transfer of thermal energy (heat) through moving matter due to the difference in densities of that matter.
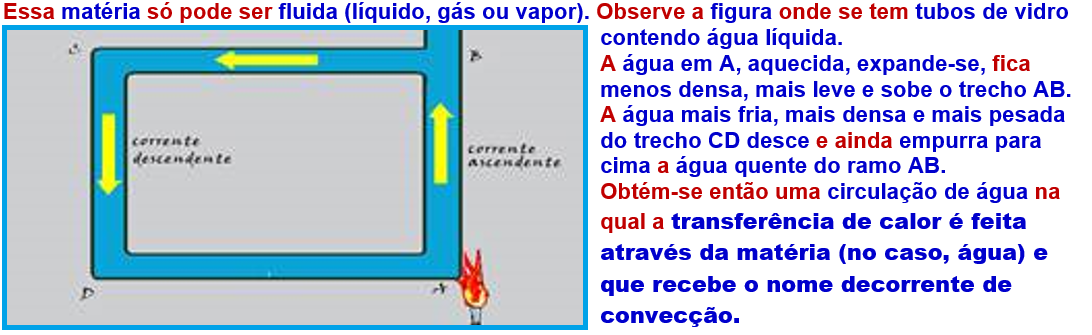
More examples:
![]()
![]()

![]()

![]()

![]()
Coastal breezes are also a consequence of convection. During the day , the land becomes warmer
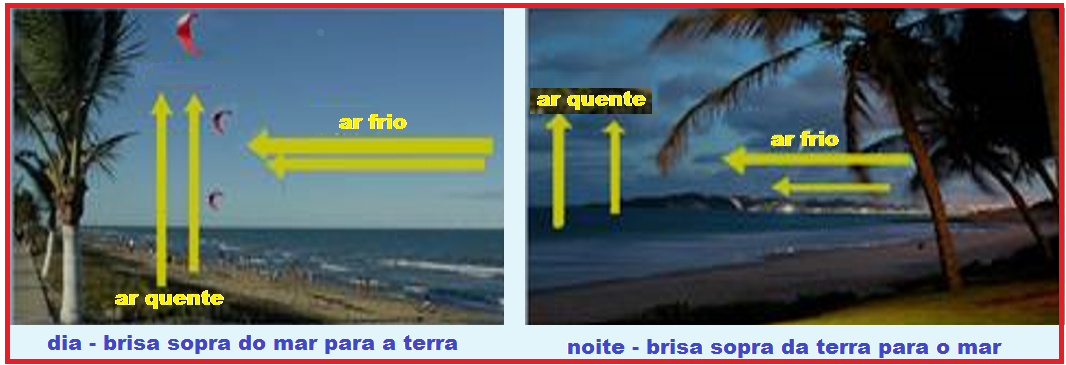
hot , the air that is close to it heats up and rises, producing a low pressure zone, “pulling” the air that is over the sea . At night the opposite occurs.
![]()
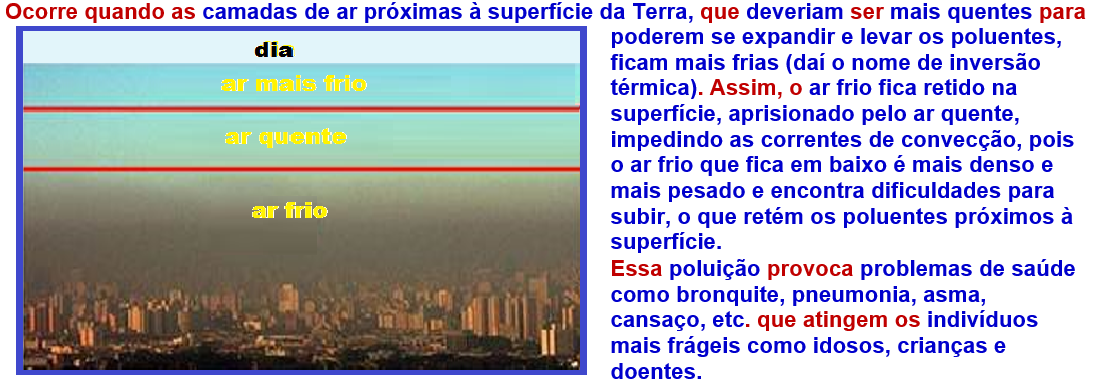
Thermal inversion
![]()
Thermal convection does not occur in a vacuum , as it requires a material medium to propagate.
Thermal conduction
Heat is conducted from one point in the body to another without the particles moving.
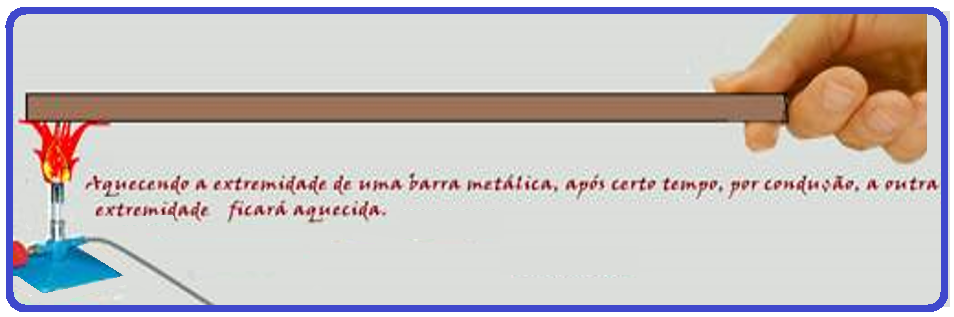
Explaining the phenomenon microscopically : the region close to the flame has the vibratory movement of its molecules increased, thus acquiring greater kinetic energy , which is transferred through shocks to neighboring particles, which also increase their vibratory movement. Through this energy transport, the entire bar is heated.
Information:
![]()
Conduction does not occur in a vacuum, as it needs a material medium (with matter ) to propagate.
![]()
Metals are good conductors of heat and are used in the manufacture of devices that allow other bodies, especially liquids, to be rapidly heated. Metals are excellent conductors of heat because their outermost electrons are “weakly” bound, making them free to transport energy through collisions through the metal .
![]()
Bodies that are poor conductors of heat : glass, rubber, styrofoam, wool, cotton, ice, some skins,
animals, gases, cork, polystyrene, ceramic fiber (composed of Alumina and Silica), glass wool (a component manufactured in a blast furnace from silica and sodium, agglomerated by synthetic resins), etc. 
These poor conductors of heat (thermal insulators) have the outermost electrons of their atoms firmly bound.
![]()
Liquids and gases , in general, are poor conductors of heat.
Air, for example, is a great thermal insulator.
Woolen clothes , animal hair, Styrofoam, and sawdust are excellent thermal insulators, as they

retain air between their fibers.
![]()
Snow is another example of a good thermal insulator. This is because snowflakes are made up of crystals, which accumulate to form fluffy layers that trap air.
Snow accumulated on the ground keeps the temperature higher than if the ground were uncovered.
Thus, in agricultural regions, it protects the roots of plants, preventing them from freezing.
In mountainous regions , snow is important to maintain or increase the flow of rivers , through its gradual melting, in spring and summer.
![]()
In cold weather , birds tend to ruffle their feathers to capture air between them and warm themselves up , becoming “plumper” and maintaining a small layer of air over their bodies .

To adapt to the cold, mammals (like us) and birds raise their fur or feathers (that is, they get goosebumps!) .
This happens because goosebumps increase thermal insulation. The more fur or feathers the animal has, the better this protection system will be .
![]()
In construction , for better thermal and acoustic insulation, it is advisable to use double walls with ceramic bricks, leaving an air gap between the walls and placing a plate composed of rigid polystyrene foam (Styrofoam) between the ceramic bricks , which is a proven insulating material, used in civil construction to save energy. It is also used in buildings because it is light, resistant, easy to operate and has a low cost.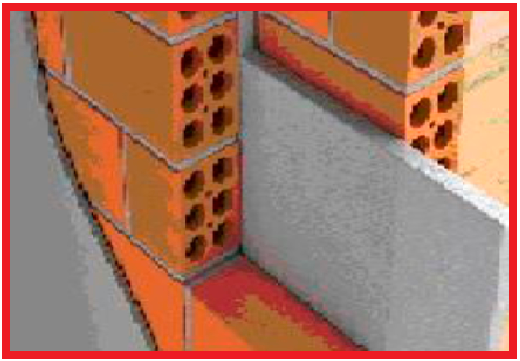
![]()
Ice is an excellent thermal insulator. Eskimos use it to build their homes, which are called igloos.

Normally the inside is lined with seal skins , making the enclosure quite comfortable.
Lighting is provided by a window made either from a block of transparent ice or from seal or whale skin.
The heat from the fire melts some of the blocks , but the water runs off and freezes again, reinforcing the seal of the ice walls.
At the entrance of the igloo, a small tunnel is built to prevent the wind from reaching the interior.
![]()
A diver wears a wetsuit that prevents the loss of body heat. Neoprene is made of a type of rubber that contains thousands of tiny bubbles inside .
Thanks to this feature, the water that enters the suit does not escape, so it is heated by body temperature and creates an insulating barrier between the diver and the liquid environment in which he is surrounded.
![]()
The Bedouins of the desert wear thick, woolen clothing (thermal insulation) so that their bodies ,

During the day, maintain an internal temperature of 36.5°C and do not receive heat from the outside environment, which can reach up to 45°C . At night, when temperatures are very low, prevent heat loss to the outside environment.
![]()


Fourier’s law
The above relationships are expressed by Fourier’s law through the equation

The constant K is called the coefficient of thermal conductivity and is a characteristic of the nature of the material that separates the two media.
Heat flux is usually expressed in calories per second (cal/s) and, as it is proportional to Φ (see formula), its value is high for good conductors (e.g. silver, K = 0.99 cal/s.cm.oC) and low for good insulators (e.g. air, K=0.000061 cal/s.cm.oC).
Information:
![]()
When the ambient temperature of a room is, for example, 28°C , and you (temperature of 36.5°C) place one hand on the door handle and the other on the wood of the door, the handle feels colder than the metal even though they are both at the same temperature , because the metal is a better conductor of heat, removing more heat from your body than the wood.
The opposite would occur if you were in a sauna where the temperature is, for example, 42°C, the metal will seem hotter to you because it now provides more heat than wood as it is a better thermal conductor.
![]()

Thermal irradiation

Information:
![]()
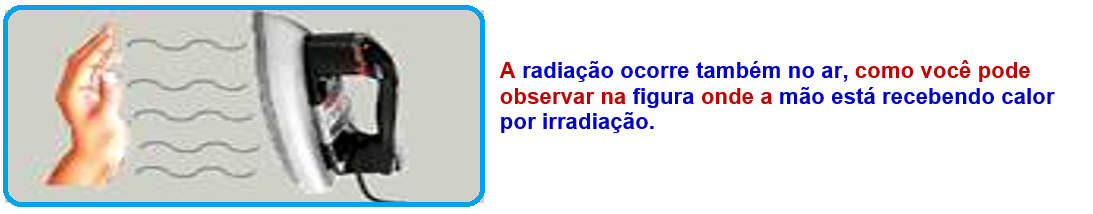
![]()
Stove
Agricultural greenhouse ![]() When radiant energy reaches the surface of a body, part of it is absorbed (about 65%), and part is reflected (about 35%).
When radiant energy reaches the surface of a body, part of it is absorbed (about 65%), and part is reflected (about 35%).
The absorbed part is retained in the body in the form of heat (thermal energy).
Direct solar radiation ( visible light radiation) is not filtered by glass or transparent plastic sheets , passing through them (refracting) easily.
This sunlight carries a large amount of energy , capable of being transmitted to the interior of the greenhouse and being retained inside and on the surface, heating them.
This process of heat conduction is known as radiation.
This happens when sunlight warms us indoors, even when the windows are closed or when we are inside the car in the sun.
The ease with which direct radiation passes through the surface of glass or transparent canvas is not repeated for the portion of reflected energy, which returns to the external environment.
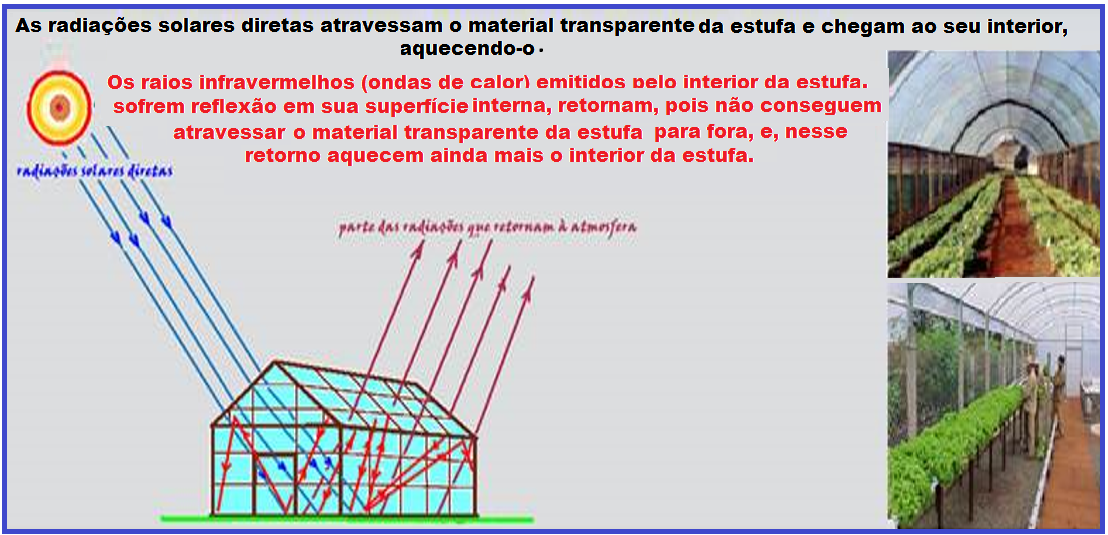
Transparent glass or canvas has a great capacity to “trap” this heat, as they are transparent to visible light and opaque to infrared radiation. This is why the inside is hotter than the outside.
![]()
When radiant energy reaches the surface of a body, part of it is absorbed , part is reflected and part is refracted.
The absorbed part is retained in the body in the form of heat (thermal energy).
When most of the energy is absorbed and a small portion is reflected or refracted, the body is called opaque , that is, it is a poor reflector and refractor. These are dark bodies, especially black bodies. Every good absorber is a good emitter.
The opposite occurs with clear and polished bodies , which are good heat reflectors, poor absorbers and poor emitters.
Examples:
![]() Pots and pans should have a black bottom to absorb more heat.
Pots and pans should have a black bottom to absorb more heat.
![]() In winter, you should preferably wear dark clothes and in summer, light clothes.
In winter, you should preferably wear dark clothes and in summer, light clothes.
What you should know, information and tips
![]()
Understand and memorize the concepts of the three heat propagation processes and the information provided.
![]()
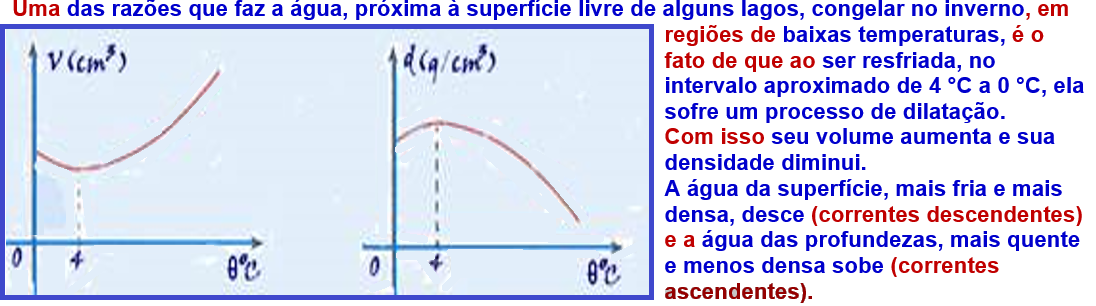
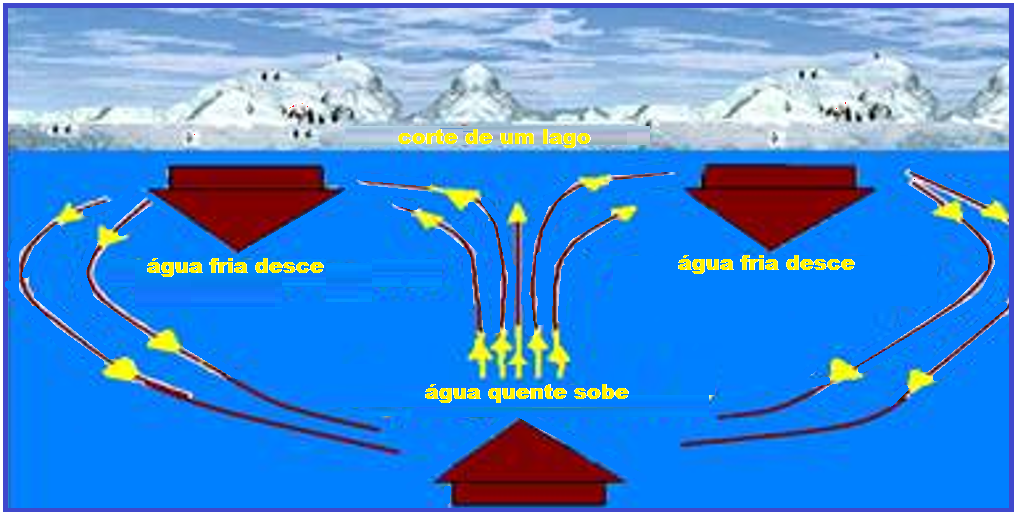
These convection currents continue their movement until all the water reaches a temperature of 4°C (maximum density ) when they cease , because all the water now has the same density.
But if the surface temperature continues to decrease below 0°C, the surface water will

freezes and this ice isolates by conduction the environment above the water surface (at less than 0oC) from the waters below it (at 4oC).
Due to this phenomenon, life is possible in the depths of lakes and seas, even when their surface is covered in ice.
![]()
Stove

![]()
The total energy emitted by radiation is proportional to the fourth power of the absolute temperature of the

issuer
![]()
Dark bodies are good heat absorbers and light, polished bodies are good heat reflectors.
![]()
Fourier’s law

![]()
Greenhouse effect
When they penetrate the Earth’s atmosphere and reach its surface, part of the light rays coming from the Sun are absorbed and transformed into heat (around 65%), others are reflected into space, but only part of these actually leave the Earth , as they are reflected back by the so-called “Greenhouse Gases” (carbon dioxide, methane, chlorofluorocarbons – CFCs – and nitrogen oxides), in the form of infrared rays (heat waves).
The effect is beneficial to planet Earth, maintaining its temperature in conditions that are conducive to life, since, without it, the temperature on Earth would be around -20°C.
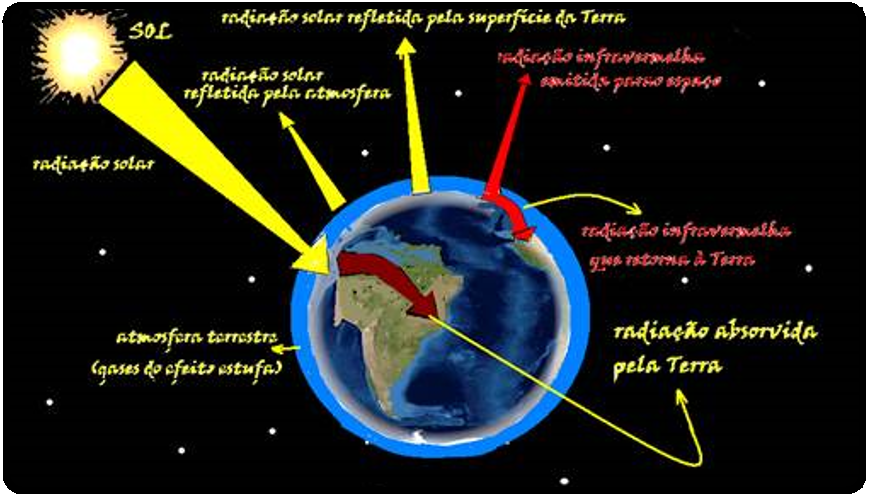
However, in recent years, the concentration of carbon dioxide in the atmosphere has increased by about 0.4% annually; this increase is due to the use of oil, gas and coal and the destruction of tropical forests.
This increase in carbon dioxide in the atmosphere allows visible radiation to escape and prevents infrared radiation (heat waves) from escaping, warming the Earth and bringing serious consequences for ocean levels and the climate.
![]()
Thermos bottle
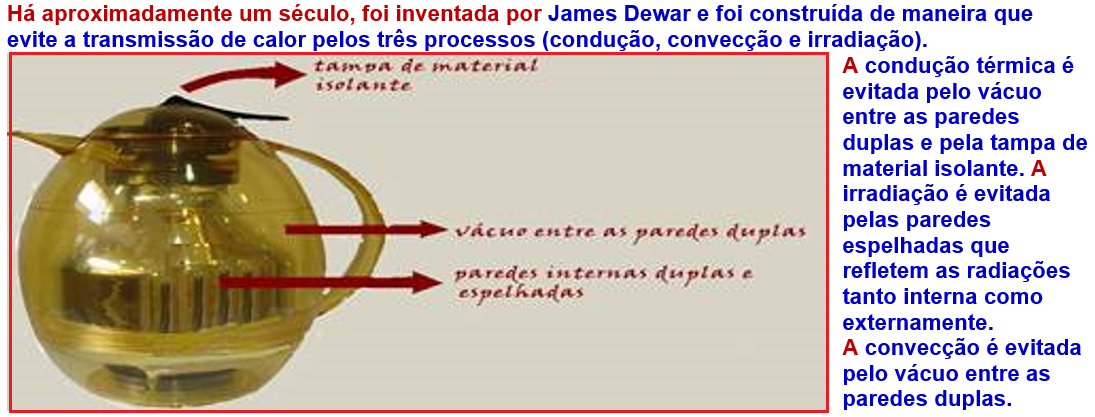
![]()
In a closed and thermally insulated room , a refrigerator, in operation, has, at a given moment, its door completely open.
Before opening this door, the temperature of the room is higher than that of the inside of the refrigerator .
After opening the door, the temperature of the room decreases, because when you open the refrigerator door, the temperature inside it is lower than that of the room’s environment and, as heat transfers from the body of higher temperature to the one of lower temperature , initially the temperature of the room will decrease.
Afterwards, with the refrigerator turned on, it starts to remove heat from inside and transfer it to the environment , and then the temperature of the room will increase.
![]()
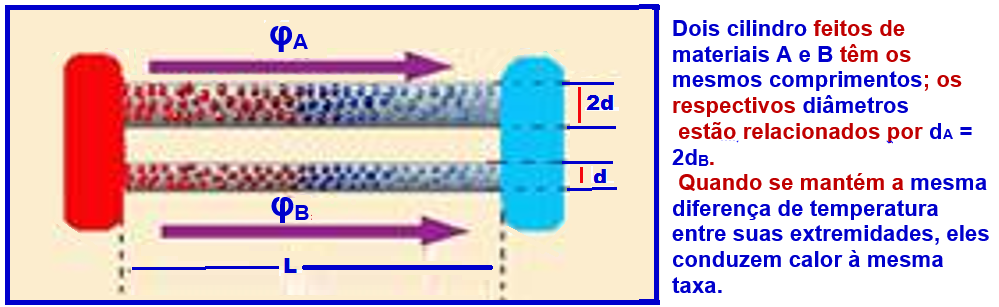
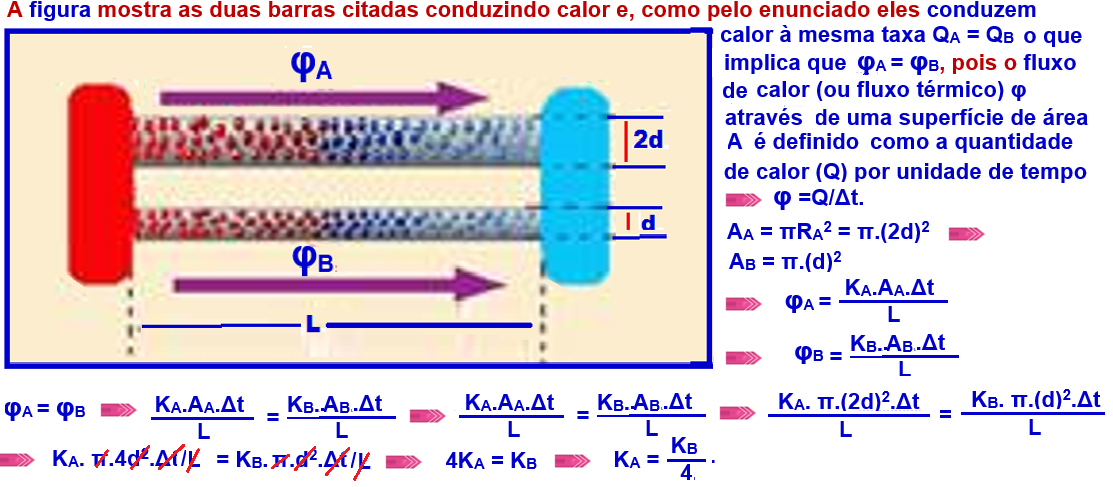
Determine the relationship between the thermal conductivities of the materials of the two cylinders .

![]()
Drying food in homemade ovens.
In this process, moisture is gradually removed due to the flow of hot air. Anyone can build a homemade food drying oven like the one below.
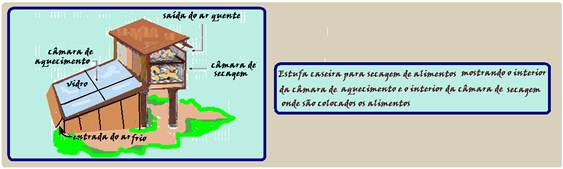
Explaining how it works: Thermal convection is the process of heat transfer that occurs thanks to the movement of a material. ![]() Note that this is exactly what happens in the example shown.
Note that this is exactly what happens in the example shown. ![]() The material that moves through the greenhouse environment is air , and the cold air is heated in the heating chamber, becomes hotter (less dense) and rises, passing through the drying chamber. The movement of the air , hotter and colder, creates the so-called convection currents.
The material that moves through the greenhouse environment is air , and the cold air is heated in the heating chamber, becomes hotter (less dense) and rises, passing through the drying chamber. The movement of the air , hotter and colder, creates the so-called convection currents. ![]()
Evaporation is the slow passage from liquid to gas , it happens more quickly with the help of wind (moving air), mainly, in this case, hot air.
![]()
There are three cylinders of the same cross-section, made of copper, brass and steel, whose lengths are, respectively, 46 cm, 13 cm and 12 cm. The cylinders are welded together , forming the Y- shaped profile , shown in the figure.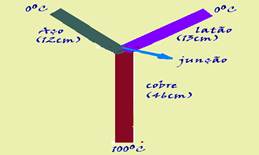
(All connected in a Y shape). The free end of the copper cylinder is kept at 100ºC , and those of the brass and steel cylinders at 0ºC. Assume that the side surface of the cylinders is thermally insulated. The thermal conductivities of copper, brass and steel are, respectively: 0.92, 0.26 and 0.12, expressed in cal.cm-1.s-1.ºC-1. In the steady state of conduction, what is the temperature of the junction?
Being the same cross section (A is the same for all) ![]() as they are thermally insulated the amount of heat and consequently the heat flux that copper (at 100oC) provides is equal to the sum of the fluxes received by steel and brass.
as they are thermally insulated the amount of heat and consequently the heat flux that copper (at 100oC) provides is equal to the sum of the fluxes received by steel and brass.
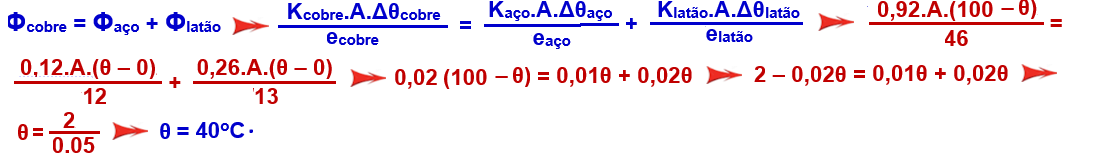
![]()
An automobile has a water mixture in its cooling system.
This mixture is pumped, circulating the heat from the engine to the radiator , where the heat is dissipated to the environment. A driver starts the engine of this car and sets off on his journey . After 10 minutes, he observes, on the temperature gauge on the dashboard , that the mixture reaches the radiator at 90°C and remains around this value during the journey. This occurs because the heat flow between two environments at different temperatures is directly proportional to the temperature difference between them . Thus, the greater the temperature difference between the radiator and the environment, the greater the heat dissipation flow.
Entrance exam exercises on this content can be found in Thermometry (heat propagation processes)
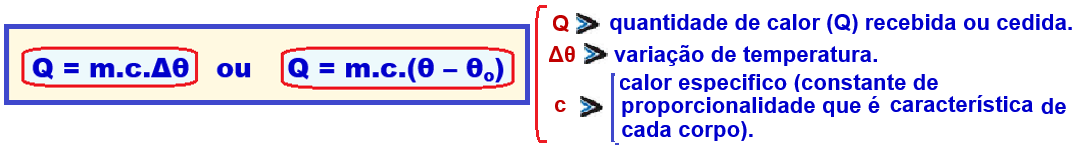
Fundamental equation of calorimetry (sensible heat or specific heat)
Sensible heat or specific heat (c) is the heat that causes a variation in the temperature of a body, without it changing its physical state.
It is experimentally verified that the amount of heat (Q) received or given off by a body is directly proportional to its mass m and its temperature variation Δθ.
Mathematically:

What you should know, information and tips
Substituting m = 1g and Δθ = 1 o C in the expression Q = mcΔθ ![]() Q=1.c.1
Q=1.c.1 ![]() Q = c
Q = c ![]() that is, the specific heat c of a substance is the amount of heat required to make the temperature of a mass of 1g of that substance undergo a temperature variation of 1 o C.
that is, the specific heat c of a substance is the amount of heat required to make the temperature of a mass of 1g of that substance undergo a temperature variation of 1 o C.
![]()
When it is stated, for example, that the specific heat of mercury is 0.033 cal/g o C , it means that, for a mass of 1g of mercury to undergo a temperature variation of 1 o C, it must receive or give up 0.033 cal.
![]()
Different substances have different specific heats; ![]() water is one of the substances that has the highest specific heat (c water = 1 cal/g o C).
water is one of the substances that has the highest specific heat (c water = 1 cal/g o C).
![]()
The greater the specific heat of a substance , the greater the amount of heat required to vary its temperature by the same value , that is, the greater the specific heat of a body, the greater its difficulty in being heated or cooled.
![]()
Graph of the amount of heat (Q) received by a body as a function of temperature θ
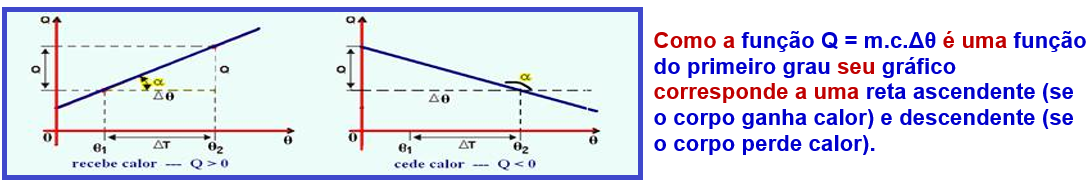
![]()
In desert regions the thermal amplitude ( difference between maximum and minimum temperatures) is quite high and in coastal regions it is much lower due to the high specific heat of water in relation to other substances. Thus, water can absorb or
give off large amounts of heat with little change in temperature, unlike desert sand which has a low specific heat.
This occurs due to the hydrogen bonds in water that hold the molecules together. The absence of water causes an environment to have a low specific heat, and thus the environment heats up easily and cools down easily.
Heat capacity (C)
The thermal capacity (C) or heat capacity of a body is defined as the product of the mass of that body by the specific heat of the substance of which it is composed, that is, ![]() C = mc
C = mc ![]() as Q = mcΔθ
as Q = mcΔθ ![]() Q = C.Δθ
Q = C.Δθ ![]() or Q = C.(θ – θ o ).
or Q = C.(θ – θ o ).

If, for example, the heat capacity of a body is C = 40 cal/ o C , this means that when that body receives or gives up 40 calories, its temperature will increase or decrease by 1 o C.

Principle of heat exchange
By placing several bodies at different temperatures inside an adiabatic container , there will be heat exchanges between them , until they reach thermal equilibrium.
Thus, as the container is adiabatic, the amount of heat given off by the hottest bodies is equal to the amount of heat received by the coldest ones .
Principle of Conservation of Energy
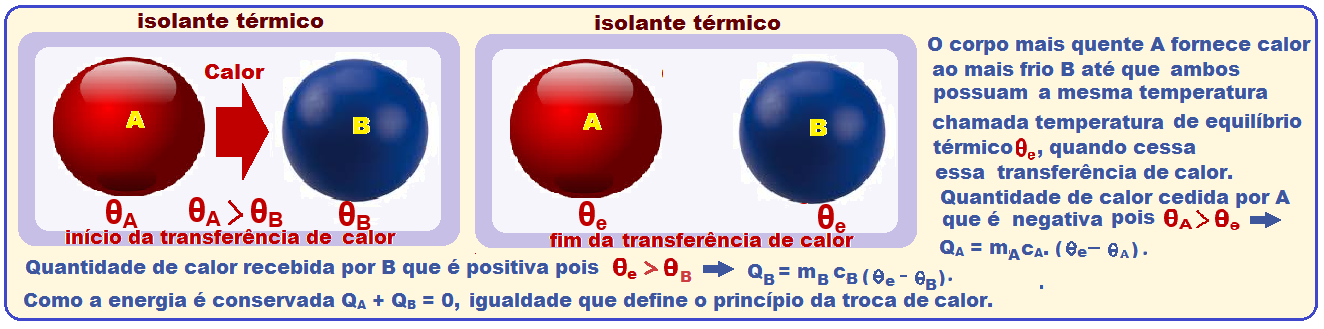
The above theory is valid for more than one body and, as the amount of heat received is positive and that given off is negative, we have ![]() Q 1 + Q 2 + Q 3 + …. + Q N = 0 or m 1 .c 1 .(θ e – θ 1 ) + m 2 .c 2 .( θ e – θ 2 ) + m 3 .c 3 .( θ e – θ 3 ) + … + m N .c N .( θ e – θ N ) = 0.
Q 1 + Q 2 + Q 3 + …. + Q N = 0 or m 1 .c 1 .(θ e – θ 1 ) + m 2 .c 2 .( θ e – θ 2 ) + m 3 .c 3 .( θ e – θ 3 ) + … + m N .c N .( θ e – θ N ) = 0.
Statement of this principle of heat exchange:

Water equivalent
The water equivalent E of a body corresponds to the mass m of water that, exchanging the same amount of heat as that body, undergoes the same temperature variation as it.
Since the specific heat of water is 1 cal/g°C, it follows that the water equivalent of a body is numerically equal to its heat capacity.
If a body has a thermal capacity equal to 40 cal/°C , its equivalent in water is 40g .
This means that the body, or the 40g of water equivalent, when receiving the same amount of heat, undergo the same temperature variation.
Q = E = m water .c water .Δθ ![]() Q = E = m water .1 . Δθ Q = E = m water .Δθ Q = C body .Δθ m water .Δθ =
Q = E = m water .1 . Δθ Q = E = m water .Δθ Q = C body .Δθ m water .Δθ = ![]()
![]()
![]()
C body .Δθ ![]() m water = C body .
m water = C body .

Calorimeters
They are adiabatic containers (they prevent heat exchange with the external environment) where heat exchange between bodies placed inside them is studied.

The calorimeter is normally used to measure the specific heat of a body of mass (m c ), by immersing it inside the calorimeter , where there is water of mass (m a ), a thermometer and a liquid stirrer.
The body whose specific heat you wish to determine is heated and introduced into the water in the calorimeter . The system is stirred and the thermal equilibrium (t e ) is reached, which is measured.
Knowing the initial temperatures of the water and the body and the specific heat of the water, the specific heat (c c ) of the body is determined by the expression (from the principle of heat exchange) m c .c c .(t e – t oc ) + m a .c a .(t e – t oa ) = 0.![]()
What you should know, information and tips

Heat capacity is a characteristic quantity of a body (its mass is involved);
Specific heat is a characteristic quantity of a substance.
![]()

Principle of heat exchange between several bodies ![]() as the amount of heat received is positive and that given off is negative, we have
as the amount of heat received is positive and that given off is negative, we have ![]() Q 1 + Q 2 + Q 3 + …. + Q N = 0 or m 1 .c 1 .(θ e – θ 1 ) + m 2 .c 2 .( θ e – θ 2 ) + m 3 .c 3 .( θ e – θ 3 ) + … + m N .c N .( θ e – θ N ) = 0.
Q 1 + Q 2 + Q 3 + …. + Q N = 0 or m 1 .c 1 .(θ e – θ 1 ) + m 2 .c 2 .( θ e – θ 2 ) + m 3 .c 3 .( θ e – θ 3 ) + … + m N .c N .( θ e – θ N ) = 0.
Statement of this principle of heat exchange:

![]()
Calorimeters
They are adiabatic containers (they prevent heat exchange with the external environment) where heat exchange between bodies placed inside them is studied.
