Física Térmica – Termodinâmica – EN
THERMAL PHYSICS – THERMODYNAMICS
Commented Resolution
Thermal Physics
Thermodynamics
01- (ENEM-MEC)
At sea level, where atmospheric pressure is normal and equals 1 atm, water in an open pan boils at 100 ° C. If the pan is closed, the water vapor that forms inside it does not evaporate.

dissipates into the environment increasing the internal pressure of the pan,
It can reach up to 2 atm, where water boils at a temperature of approximately 120 ° C. At this pressure, water boils at a temperature approximately equal to 120 ° C. Therefore, food cooks in less time. Pressure cookers have safety valves that operate when the pressure reaches a dangerous point — R- B
02- (ENEM-MEC)
If a certain mass of water is vaporizing, under normal pressure, the phenomenon is occurring at 100 ° C and, if you increase the size of the flame, the temperature of the water will continue to be 100 ° C, only the vaporization will be faster — R- E
03-(ENEM-MEC)
At a temperature of -55 ° C, water on Mars would be in a solid state — R- E
04-(ENEM-MEC)
When you pull the plunger you are increasing the volume of the region where the water is, causing a decrease in pressure and consequently the boiling temperature decreases, causing the water to boil at a temperature lower than 100oC — R- D
05-(ENEM-MEC)
It is a transformation of heat (generated by the burning of fuel) into work (movement of the vehicle) — A- A
06-(ENEM-MEC)
I. Correct — empty spaces between shelves facilitate convection currents.
II. False — the ice mass acts as a thermal insulator, preventing the freezer from removing heat from inside the refrigerator .
III. Correct — the heat removed from inside the refrigerator must exit through the “grid”.
R-D
07-(FGV-SP)
In isobaric transformations, the pressure remains constant, thus varying the volume and temperature. If the temperature varies, the internal energy varies, which invalidates option A.
In isometric transformations, the volume remains constant, and therefore, pressure and temperature will vary, which invalidates option B.
There is no work, in fact, if the volume does not vary, in isometric or isovolumetric transformations. Which invalidates option D.
In isometric transformations only the volume remains constant, the pressure and temperature vary, which invalidates option E.
Only in option C do we have a correction, because in fact in adiabatic transformations there is no heat exchange between the gas and the container, in addition to the external environment, of course.
R-C
08-(UEL-PR) Read the following text.
In a refrigeration system, such as a refrigerator or air conditioner, work is done so that heat from the cold source is transferred to the hot source — R- D
09-(UEL-PR)
I. Correct — The refrigerator is a heat engine that transfers heat from the cold source to the hot source.
II. Correct — The refrigerator is a heat engine that uses a refrigerant liquid with condensation and vaporization processes.
III. False — the higher the latent heat of vaporization of the refrigerant, the less work is done by the refrigerator.
IV. Correct — The refrigeration process performs work through a compressor that maintains the circulation of the refrigerant fluid, removing heat from the cold source and transferring it to the hot source.
R-D
10-(UFSCAR-SP)
I. True — see theory
II. False — as the temperature varies throughout the cycle, internal energy will also vary
III. False — it is impossible to transform all received heat into work
A- A
11-(UFRN-RN)
The higher the speed at which the molecules are agitated, the easier it will be for them to escape — R- C
12-(UFMS-MS)
Since the volume is constant, the piston does not rise or fall, doing no work on the external environment, or W=P.(V2 – V1) — V1=V2 — W=0 — R- C
13-(UEG-GO)
It is possible for a gas to receive heat and its temperature not to vary as long as it is an isothermal transformation where all the heat received is transformed into work, since, being ΔU=0 — Q=W — R- E
14-(UEL-PR)
Read the theory below:
The curves in the graph below are called state diagrams or phase diagrams. In it, for a given substance:
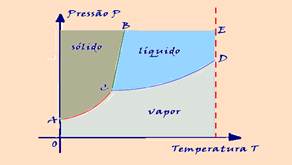
![]() CB curve — provides the temperature and pressure of melting or solidification and this curve represents the melting curve where the solid and liquid states are in equilibrium.
CB curve — provides the temperature and pressure of melting or solidification and this curve represents the melting curve where the solid and liquid states are in equilibrium.
![]() CD curve — provides the temperature and pressure of vaporization or condensation and this curve represents the vaporization curve where the liquid and vapor states are in equilibrium.
CD curve — provides the temperature and pressure of vaporization or condensation and this curve represents the vaporization curve where the liquid and vapor states are in equilibrium.
![]() AC curve — provides the temperature and pressure of sublimation or crystallization and this curve represents the sublimation curve where the solid and vapor states are in equilibrium.
AC curve — provides the temperature and pressure of sublimation or crystallization and this curve represents the sublimation curve where the solid and vapor states are in equilibrium.
In this graph, point C , common to the three curves, is called the triple point and, at it, the substance is in equilibrium in the three states: solid, liquid and vapor.
A- A.
15-(UNIFENAS-MG)
See theory below:
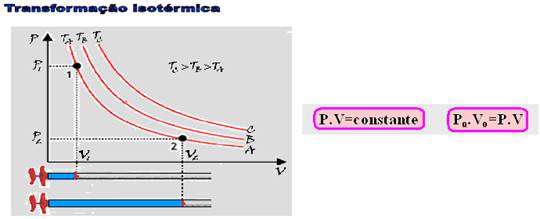

R-C.
16-(FUVEST-SP)
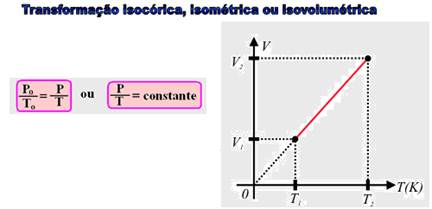
You may have noticed how difficult it is to open the freezer door right after closing it, requiring you to wait a few

seconds to open it again. This is because, if the freezer door is left open for some time, part of the air inside it is replaced by warmer air from outside. After you close the refrigerator door , the internal temperature of the air inside it will decrease and, as this is an isovolumetric transformation, the pressure also decreases (P/T = constant), causing the external pressure to be greater than the internal pressure, making it difficult to open the door. However, after a few moments, air enters through the refrigerator’s sealing system, reducing the difference between the external and internal pressures, thus making it easier to open the door.
Isovolumetric — P o /T o =P/T — P o /(27 + 273)=P/(273 – 18) — P=(255/300)P o — P=0.85P o — P=85% of initial pressure — R- D
