Física Térmica – Resolução – EN
THERMAL PHYSICS – RESOLUTION
Commented Resolution
Thermal Physics
Dilatometry
01-
I- In the hottest time, the volume increases and, in the same volume there would be less mass — you would suffer a loss — False
II- With a lower temperature, the volume decreases and, in the same volume there would be more mass — you would have an advantage — correct
III- Correct — you would be buying what really matters, which is the dough.
R- And
02-
In each liter of hydrated alcohol there are — 0.96L of alcohol (96%) and 0.04L of water (4%) — calculation of the mass of alcohol — dalcohol=malcohol/Valcohol — 800g/L=malcohol/0.96L — malcohol=768g — mass of water — dwater=mwater/Vwater — 1,000g/L=mwater/0.04L — mwater=40g — dmixture=(malcohol + mwater)/Vmixture=(768 + 40)/1 — dmixture=808g/L — R- E
03-
Volumetric or cubic expansion occurs when, due to a rise in temperature, a body undergoes an increase in its three dimensions (volume).
All laws valid for linear and superficial expansion are also valid for volumetric expansion, that is:

ΔV – volumetric expansion — V o – initial volume — γ – average coefficient of volumetric expansion — Δt – temperature range
The volumetric variation ΔV of the 20 thousand liters of alcohol when subjected to a temperature variation (Δθ) of 30°C is given by — ΔV = V o. λ.∆t — γ=1.10 -3 o C -1 — ∆V=20,000 liters=20.10 3 liters — ∆t=30 o C — ∆V=20.10 3 .
10 -3 .30 — ∆V=600 liters — this variation in liters of alcohol is in one day, in one week you will have — ∆V total =7×600 — ∆V total =4,200 liters — this volume of alcohol is sold at R$1.60 per liter, with no purchase cost — the financial gain GF due to heating the alcohol after one week is worth — GF = 4200×1.60 = R$6,720.00 —
R-D.
04-
Analyzing the graph, we notice that the specific volume decreases from 0 °C to 4 °C, increasing from that temperature onwards.
Approximating the values read on the graph, we find a reduction from 1.00015 cm 3 /g to 1.00000 cm 3 /g from 0 °C to 4 °C, that is, 0.00015 cm 3 /g. This represents a percentage reduction of 0.015%, which is less than 0.04% — R- C —
Observation on this subject that has Enem characteristics — one of the reasons why water, close to the free surface of some lakes, freezes in winter, in regions with low temperatures, is the fact that when it is cooled, in the approximate range of 4 °C to 0 °C, it undergoes a process of expansion.
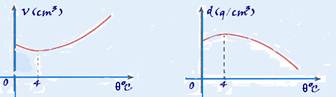
As a result, its volume increases and its density decreases. The surface water, which is colder and denser, sinks (descending currents) and the water in the depths, which is warmer and less dense, rises (ascending currents).
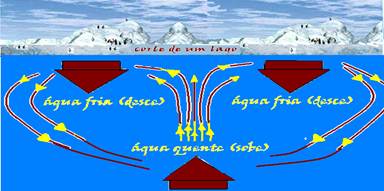
These convection currents continue their movement until all the water reaches a temperature of 4 ° C (maximum density) when they stop, because all the water now has the same density.
But if the surface temperature continues to decrease below 0 ° C, the surface water freezes and this ice insulates by conduction the environment above the water surface (at less than 0 ° C) from the water below it (at 4 ° C).
Due to this phenomenon, life is possible in the depths of lakes and seas, even when their surface is covered in ice.
05-
Since A is fixed, the dimensions of the system must increase, as it is being heated and point B must move away from A (see the figure where red represents the expanded bar)
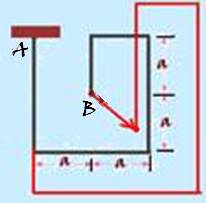
R-B
06-
Since the bar is made of the same material (same expansion coefficient) and undergoes the same temperature variation, all of its points will undergo the same expansion and it will not be deformed —
A- A
07-
The expansion of aluminum is twice that of steel — see figure
R- And
08-
ΔL = Lo. α. Δθ — ΔL = 30 . (11. 10-6). (40 – 10) = 99 . 10-4 m — R-C
09-
Since the coefficient of linear expansion of brass is greater than that of steel, brass expands more when heated and contracts more when cooled — R-C
10-
The higher the coefficient of expansion, the more the body expands when heated and the more it contracts when cooled.
I. A expands more than B — Correct
II. False — see (01)
III. The gap will decrease — False
IV. They have different L o — False
V. Only the plate will expand — Correct
VI. Only the plate will expand — Correct
A- A.
11-
ΔV=0.006V o — ΔV=V o .γ.Δt — 0.006V o =V o. γ.200 — γ=3.10 -5 o C -1 — α/1=γ/3 — α/1=3.10 -5 /3 — α=1,0.10 -5 o C -1 — R- A
12-
The part of the inner surface of the glass expands more than the part of the outer surface — R- C.
13-
Note in the graph that if you decrease the temperature of the system, the volume of the container becomes smaller than that of the water and it overflows and that if you increase the temperature of the system, the volume of the water becomes larger than that of the container, and it overflows — R- C
14-
Please pay close attention to the following explanations: The bottle in the figure below contains a “thief” with the liquid filling the container up to its level (figure I).
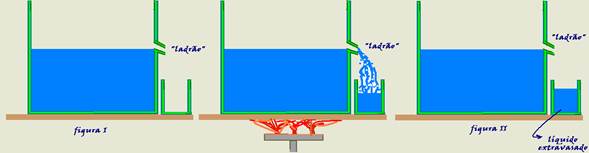
After the set is heated by Δt, as liquids normally expand more than solids, part of the liquid leaks through the “thief” (figure II).
This volume of extravasated liquid corresponds to the apparent expansion of the liquid (ΔV ap ), initial volume V o and apparent volumetric expansion coefficient (γ ap ) and not to the real expansion of the liquid, since the bottle also expands.
R-C.
15-
Note that the value of the coefficient of expansion of aluminum is twice the coefficient of expansion of concrete and, as they have the same initial dimensions and undergo the same temperature variation, the aluminum sheet expands twice as much as the concrete sheet — R- E
16-
All are correct, except IV which is wrong because, from the expression ∆V=Vo.λ.∆θ you can see that the coefficient of thermal expansion (λ) is directly proportional to the expansion (∆V) —
R- E.
